Sodium Hyaluronate - 500 kDa
- Article
- Sodium Hyaluronate - 500 kDa
- Product Number
- H-0500-250MG
- Supplier
- Echelon Biosciences
- Size
- 250 mg
- Description
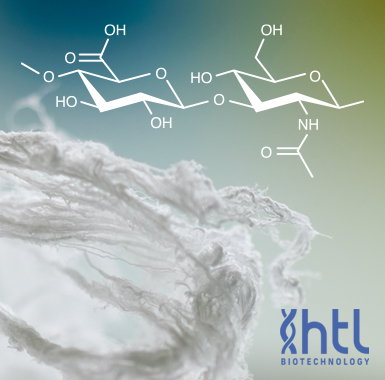
- Sodium Hyaluronate is the sodium salt of hyaluronic acid (HA) also known as hyaluronan a glycosaminoglycan consisting of D-glucuronic acid and N-acetyl-D-glucosamine disaccharide units. HA is one of several glycosaminoglycan components of the extracellular matrix of connective tissue. HA is a naturally occurring biopolymer involved in numerous biological processes including tissue hydration and structural scaffolding. HA is increasingly used as a reagent and investigated in medical pharmaceutical and bioengineering applications. Its use as a reagent includes hydrogels for use in aesthetics ophthalmology rheumatology urology wound healing and 3D bioprinting.Our medical grade Sodium Hyaluronate is produced by HTL by fermentation of a Streptococcus equi strain (Group C of the Lancefield Classification / Non GMO / Without any material from animal origin). HTL's proprietary process allows the production of Sodium Hyaluronate fiber with an exceptionally low level of impurities. HTL Sodium Hyaluronate raw material is a medical grade pharmaceutical product manufactured under cGMP conditions and is covered by a Certificate of Suitability of Monographs if the European Pharmacopoeia (CEP) and Drug Master File (DMF).HTL Sodium Hyaluronate is aliquoted and distributed by Echelon for research use only.MW range: 400-600 kDa (see Certificate of Analysis for lot specific MW)Storage: dry product at 5 °C protected from light and humidity. Solutions should be stored frozen at -20 °C or below.About HTL: HTL is the world leader in the production of pharmaceutical grade Sodium Hyaluronate by fermentation. www.htlbiotech.comBulk discounts available please email echelon@echelon-inc.com for information.References1) M.A. Serban A. Skardal (2018) oeHyaluronan chemistries for three-dimensional matrix applications Matrix Biology 78-79 337-3454. doi: 10.1016/j.matbio.2018.02.010.2) C.B. Highley G.D. Prestwich GD Burdick JA. (2016) oeRecent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol. 40 35-40. doi: 10.1016/j.copbio.2016.02.008.3) J.A. Burdick G.D. Prestwich (2011) oeHyaluronic acid hydrogels for biomedical applications. Adv Mater. 23 H41-56. doi: 10.1002/adma.201003963.4) A. Dodero R. Williams et al. (2019) oeA micro-rheological and rheological study of biopolymers solutions: Hyaluronic acid Carbohydrate Polymers 203 349-355 doi:10.1016/j.carbpol.2018.09.072.5) P.A. Simmons J.G. Vehige (2017) oeInvestigating the potential benefits of a new artificial tear formulation combining two polymers. Clin Ophthalmol. 11 1637-1642. doi:10.2147/OPTH.S135550.
- Category
- Lab Reagents & Chemicals
- Product Group
- Biochemicals
- Shipping Temperature
- RT
- Supplier URL
- Visit supplier product page